Bowel cancer remains one of the most common and deadly cancers in Australia. However, recent advancements in early detection, surgical techniques, molecular diagnostics, and personalised therapy are transforming how it is managed.
According to the American Cancer Society (ACS), bowel cancer is the third most diagnosed cancer in both men and women and the second leading cause of cancer death when men and women are combined.
Historically viewed as a disease of older adults, bowel cancer is increasingly being diagnosed in younger individuals, prompting a critical shift in screening recommendations. Both the ACS and the United States Preventive Services Task Force now recommend routine bowel cancer screening beginning at age 45 for average-risk individuals. With innovations ranging from liquid biopsies to targeted and immune-based therapies, bowel cancer is becoming a model for personalised oncology.
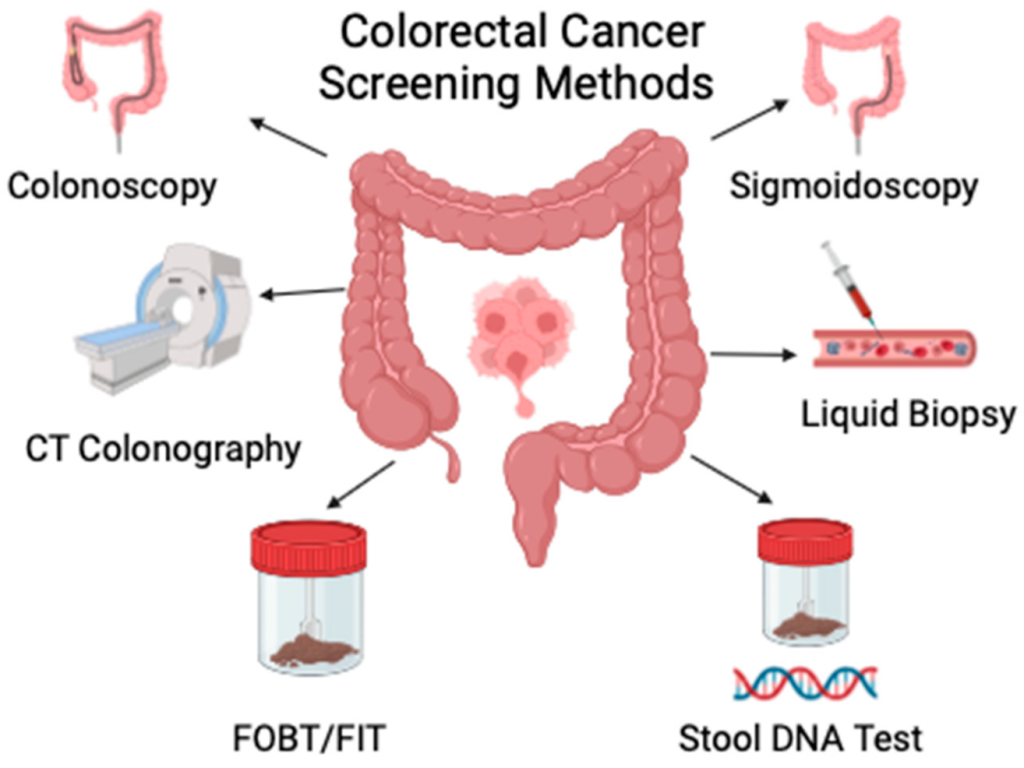
1. Non-invasive Screening and Diagnostic Tools are Expanding Access to Early Detection
Advances in bowel cancer screening are enhancing detection rates and improving accessibility and patient adherence. Stool DNA tests, such as Cologuard, (In Australia: Australian National Bowel Cancer Screening Program) identify abnormal DNA and blood in stool with a sensitivity of 92% for bowel cancer. Although not a replacement for colonoscopy, they provide a non-invasive alternative that is especially useful in average-risk populations.
Newer modalities are also making an impact. The Shield blood test, approved by the FDA in 2024, detects cancer-related biomarkers in blood and can be used as a primary screening option. Tests like ColoHealth detect methylated Septin9 DNA in blood, and ColoSense examines RNA markers in stool, each offering a different biological snapshot of tumour presence.
These tools not only support early diagnosis but also enable surveillance after treatment. As the field moves towards more personalised, risk-adapted screening, a combination of molecular and imaging-based approaches may become the norm for bowel cancer detection and management.
2. Minimally Invasive Surgical Techniques and Adaptive Radiation are Improving Outcomes
Surgery remains the cornerstone of curative-intent treatment for localised colorectal tumours, and recent innovations have made procedures safer and more precise. Robotic-assisted surgeries enable smaller incisions, enhanced dexterity in confined anatomical spaces, reduced postoperative pain, and shorter recovery times compared with open procedures. Similarly, laparoscopic approaches remain widely used and offer significant reductions in hospital stays and complications.
In rectal cancer, radiation therapy plays an important role in neoadjuvant and adjuvant settings. Adaptive radiation therapy is an emerging technique that adjusts radiation delivery in real time based on daily imaging of anatomical changes, which may improve targeting accuracy and reduce exposure to healthy tissue. The integration of these technologies supports organ preservation strategies and may reduce the need for permanent colostomies in select cases of rectal cancer.
3. Genomic Profiling is Redefining Treatment Decisions in Advanced Bowel Cancer
Genomic profiling has become a critical component of the diagnostic and therapeutic strategy for patients with advanced or metastatic bowel cancer. Testing for mutations such as KRAS, NRAS, BRAF, HER2, and NTRK—as well as markers of high microsatellite instability (MSI-H) and mismatch repair deficiency (dMMR)—enables oncologists to tailor therapy to the molecular makeup of a patient’s tumour.
For example, patients with MSI-H/dMMR tumours are highly responsive to immunotherapy, whereas patients with KRAS or BRAF mutations may benefit from the use of specific targeted agents. Next-generation sequencing technologies enable broad-panel testing from a single biopsy or liquid biopsy sample, thereby enhancing the ability to detect resistance mutations that inform treatment changes over time.
4. A Growing Arsenal of Targeted Therapies is Transforming the Treatment of Metastatic Bowel Cancer
Targeted therapy has evolved dramatically with the identification of actionable genetic alterations. For patients with KRAS G12C mutations, agents such as adagrasib and sotorasib have shown promise when used in combination with epidermal growth factor receptor (EGFR) inhibitors. Antiangiogenic therapies targeting vascular EGF/ vascular EGFR—such as fruquintinib, bevacizumab, and ramucirumab—continue to play an important role in later lines of treatment by disrupting the blood supply to the tumour.
Patients with BRAF V600E mutations benefit from combination regimens such as encorafenib plus cetuximab. Likewise, HER2-positive tumours can be treated with trastuzumab-based regimens. For those with rare alterations, such as NTRK or RET fusions, tissue-agnostic therapies like larotrectinib and entrectinib have shown high response rates. Collectively, these advances underscore the importance of comprehensive biomarker testing in guiding the selection of therapy.
5. Immunotherapy Offers Durable Responses in Select Bowel Cancer Subtypes
Immunotherapy has revolutionised treatment in a subset of bowel cancer patients with tumours characterised by MSI-H or dMMR, typically accounting for 4%-5% of metastatic cases. Agents such as nivolumab, ipilimumab, and pembrolizumab are FDA-approved for use in this setting, offering durable disease control and, in some cases, complete responses.
A landmark phase 2 trial conducted at Memorial Sloan Kettering showed that immunotherapy alone (e.g., dostarlimab-gxly) could eliminate localised rectal tumours in patients with dMMR/MSI-H disease, allowing them to avoid surgery, radiation, and chemotherapy altogether. All patients in the trial remained disease-free at follow-up, and this approach has since received FDA breakthrough designation. These findings suggest a paradigm shift in early-stage treatment for select patients with biomarker-positive conditions, highlighting the potential of genomic stratification.
Reference: Mannucci A, Goel A. Stool and blood biomarkers for colorectal cancer management: an update on screening and disease monitoring. Mol Cancer. 2024 Nov 19;23(1):259. doi: 10.1186/s12943-024-02174-w. PMID: 39558327; PMCID: PMC11575410.